|
Research Article
A randomized open-label clinical trial to evaluate convalescent plasma compared to conventional standard care management for SARS-CoV-2 (COVID-19) in hospitalized patients in the Dominican Republic
1 Research Manager, Instituto Nacional de Investigaciones de Enfermedades Infecciosas (INIEICONT), Santo Domingo, Dominican Republic
2 Blood Bank Director, Hospital General de la Plaza de la Salud Santo Domingo, Dominican Republic
3 Research Director, Hospital General de la Plaza de la Salud Santo Domingo, Dominican Republic
4 Senior Medical Manager, Macopharma SAS Medical Affairs, Atlanta, GA, USA
5 Research Director of the Faculty of Health Sciences, Universidad Autonoma de Santo Domingo, Santo Domingo, Dominican Republic
6 Universidad Autonoma de Santo Domingo, Santo Domingo, Dominican Republic
Address correspondence to:
Angiolina Camilo Reynoso
MD, PhD, Instituto Nacional de Investigación de Enfermedades Infecto-Contagiosas (INIEICONT), C/Colon No. 2, Santa Bárbara, Santo Domingo, D.N.,
Dominican Republic
Message to Corresponding Author
Article ID: 100071Z02AC2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Reynoso AC, Sosa S, Mejia D, Frontier L, Diaz A, Montero R. A randomized open-label clinical trial to evaluate convalescent plasma compared to conventional standard care management for SARS-CoV-2 (COVID-19) in hospitalized patients in the Dominican Republic. Int J Blood Transfus Immunohematol 2022;12:100071Z02AC2022.ABSTRACT
Aims: The potential benefit of blood therapy in the pandemic is an opportunity for breakthrough therapy. Still, it is also a test for countries to understand their blood supply system and its regulatory infrastructure to succeed in a potentially life-saving alternative for emergent pandemics.
Methods: A multicenter, randomized, zero-phase exploratory study is to compare the efficacy and safety of Convalescent Plasma (CP, test group) with standard care therapy (control group) in hospitalized patients for SARS-CoV-2 (COVID-19).
Results: Thirty-five patients were randomized; 18 (51.4%) corresponded to the treated group. There was a clinical improvement over time for both groups (p < 0.001), but CP didn’t show a significantly different result than standard treatment for non-critically ill patients infected with COVID-19 in the Dominican Republic (p = 0.058). The appearance of dyspnea, the increase of >50% of pulmonary infiltrates between 24 and 48 hours of evolution, and positive qualitative polymerase chain reaction (PCR) results improved significantly in the control group. Therefore, convalescent therapy presented a significant recovery in these signs and symptoms. No adverse events were reported.
Conclusion: This prospective study serves as a pilot to propose an investigation with a representative sample to evaluate the benefits of this therapy’s effects in a bigger population. The most significant advantage of Convalescent Plasma (CP) therapy was obtained in the first six days, where the improvement in clinical categorization was faster, suggesting CP is best for early and mild cases of COVID-19. Actions for enrolling volunteers for this study were not productive, an opportunity for the Dominican Republic health authorities to improve their blood donation strategies and support blood availability.
Keywords: Convalescent plasma, COVID-19, SARS-CoV-2, Dominican Republic
Introduction
The last decade saw the outbreak of many life-threatening human pathogens such as Nipah, Ebola, Chikungunya, Zika, Middle East Respiratory Syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), and more recently, a new coronavirus (2019-nCoV, SARS-CoV-2, and COVID-19). At the beginning of the 20th century, convalescent plasma (CP) was used to stop outbreaks of viral diseases such as polio, measles, mumps, and influenza. Retrospective studies of patients infected with the H1N1 influenza virus during the 1918 pandemic suggested that those treated with CP had lower mortality. However, the studies conducted do not meet many of the modern criteria for evidence-based medicine [1].
There is presently no approved medication for COVID-19, and initial trials for CP show only minor benefits. However, studies to this point are inconclusive, and randomized trials are still evolving to determine CP efficacy and protection in the present COVID-19 pandemic [2]. Similar studies during the SARS epidemic revealed better results with early initiation of CP therapy in recovered patients [3].
The lack of effective therapy for SARS-CoV-2, coupled with an overwhelming number of cases, drives the use of convalescent plasma. Reviews of recently published studies and the historical use of CP suggest that CP therapy may be considered an investigational therapy in critically ill patients [4].
Due to a history of positive results obtained during treatment of other viruses, the United States Food and Drug Administration (FDA) considered CP a promising option and authorized it to be used in COVID-19 patients facing severe or life-threatening symptoms [5]. However, such case reports and case series involving randomized control trials require further substantiation for CP therapy to aid in the prevention of the current COVID-19 infected population [6].
There is no documented experience of CP therapy in the Dominican Republic. At the same time, there is no international consensus on the optimal timing of CP administration and the possibility of fractionating plasma. The success of the application of CP therapy depends on the availability of plasma.
The availability of plasma and the level of donation intent of a recovered patient have not been reported for the Dominican Republic. From a financial and logistical perspective, health authorities and government institutions should endeavor to promote the safety and efficacy of CP therapy through increasing CP therapy clinical research, early COVID-19 patient stages of plasma collection, and investigative laboratory testing [7].
Case studies of CP use to treat COVID-19 are encouraging, particularly with a lack of alternative effective antiviral therapies. Depending on the specific goals of the individual trials, outcomes vary. Controlled-arm studies are complex, considering that the administration of the standard plasma as the control will consume valuable blood products and expose patients to potentially immunogenic antigens. Hence, comparing the standard of care is more feasible [8].
Despite the potential benefits of passive antibody therapy, there have been few concerted efforts for blood components as the first therapies against emerging infectious and pandemic threats. The potential benefit of blood therapy in the pandemic is an opportunity for breakthrough treatment. Still, it is also a test for countries to review their blood supply systems—and regulatory infrastructure—in hopes of succeeding in the discovery of life-saving alternatives for any future emergent pandemics [9],[10].
Plasma administration is expected to be safe, reduce viral load, and improve clinical outcomes. Evidence of CP as an ancestral beneficial therapeutic tool in viral infections offers a strong precedent for this therapeutic approach. It is essential to carry out controlled clinical trials with complete ethical, scientific, and methodological rigor to confirm the efficacy and safety of CP in people affected with COVID-19, thus benefiting rational decision-making based on the scientific evidence.
MATERIALS AND METHODS
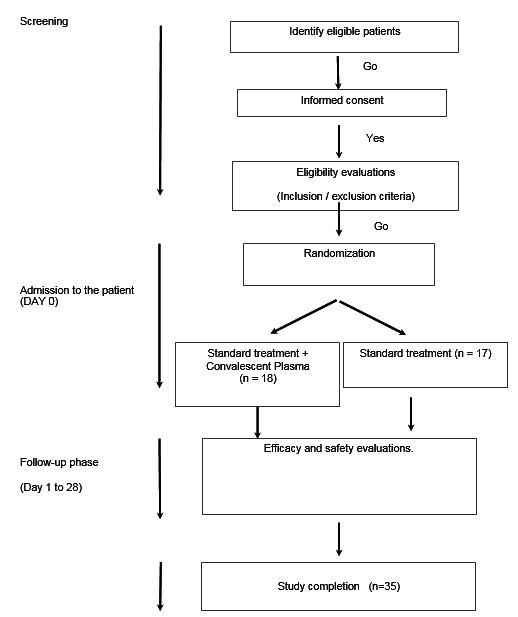
A multicenter, randomized, zero-phase exploratory study was designed to compare the efficacy and safety of CP (test group) with standard care therapy (control group) in hospitalized adult patients (≥18 years) for the infection of SARS-CoV-2 (COVID-19) with an early onset that does not require mechanical ventilation (invasive or non-invasive), but with risk factors for the development of complications. All patients received treatment following the local Protocol for the Diagnosis and Treatment of Coronavirus (COVID-19) [11]. Eligible patients were randomized to receive a single CP unit (250–300 mL). The follow-up for each patient was four weeks (Figure 1).
This trial began in the National Police General Teaching Hospital (Hospital General Docente De La Policía Nacional) of the Dominican Republic, a Military Hospital Dr. Ramón De Lara—FARD, and the General Health Plaza Hospital (Hospital General De La Plaza de la Salud—HGPS), then was widened to include other hospitals treating patients with COVID-19 in the Dominican Republic. The control arm corresponded to the research subgroup that did not receive a CP transfusion despite receiving the therapy authorized by the Ministry of Public Health (Ministerio de Salud Publica—MSP) and the HGPS. The intervention group corresponded to the research subgroup that received a single unit of CP transfusion in the first three days of hospital admission.
The reports included all adverse events, especially for serious adverse events considered potentially related to the administration of COVID-19 convalescent plasma. A consecutive series questionnaire was developed to confirm inclusion and exclusion criteria and facilitate manual data collection, later transcribed to digitize the information.
Patients were eligible for the trial if they met the following inclusion criteria: Patients requiring hospitalization for SARS-CoV-2 without mechanical ventilation (invasive or non-invasive, including oxygen mask with reserve bag) and at least one of the following: radiographic evidence of pulmonary infiltrates by imaging [chest X-ray, computed tomography (CAT) scan, etc.] or clinical evaluation (evidence of rales/crackles on examination) and oxygen saturation (SpO2) ≤ 94% in room air requiring supplemental oxygen, SARS infection-Laboratory confirmed CoV-2 as determined by polymerase chain reaction (PCR) on nasal/oropharyngeal swabs or any other relevant specimen <72 hours prior to randomization, not more than 72 hours (3 days) of hospitalization prior to administration of CP therapy (except days after initial admission to hospital for other reasons and before SARS-CoV-2 infection), no more than 10 days between onset of symptoms (fever or cough) and the day of administration of therapy or demonstration of the absence of anti-SARS-CoV-2 antibodies (patients with more than 10 days of symptoms only included, if a negative result results antibody test has been confirmed) and that they were ≥ 18 years of age.
Both groups of patients hospitalized for SARS-CoV-2 infection were categorized on an ordinal scale followed by the duration of the investigation, evaluating the proportion of patients in the categories and their progression. This proportion of patients will be compared between the arms of the study, according to the ordinal scale described below, and as published in different human study protocols approved for patients with COVID-19 in the Cochrane study record NCT04622865 and the ClinicalTrials.gov Identifier NCT04622865. Ordinal scale: (1) not hospitalized, without limitations in activities. (2) Not hospitalized, limitation of activities. (3) Hospitalized, requiring no supplemental oxygen. (4) Hospitalized, requiring supplemental oxygen. (5) Hospitalized, with non-invasive ventilation or high-flow oxygen devices or oxygen mask with reserve bag. (6) Hospitalized, with invasive mechanical ventilation or ECMO. (7) Death. No international standardized protocol was in place when creating this trial protocol. Regarding the transfusion, the extraction was carried out, ensuring that it did not exceed 800 mL of collection per session (15% of the volume of an average individual). The total volume was divided into bags of 200 mL.
Human experiments
The Ethics Committee of the National Institute for Infectious-Contagious Diseases Research (INIEICONT) of the Dominican Republic, the Research Ethics Committee of the HGPS, and the National Council of Bioethics in Health, CONABIOS, registered with No. TRB00003833 and Guarantee No. FWA000061108 in the International Review Board Information and Office of Human Research Protection approved this research protocol under No. 013-2020. This project was also possible thanks to the collaboration with the Autonomous University of Santo Domingo (UASD) and the Ministry of Public Health (MSP) of the Dominican Republic.
Statistics
The data was processed using IBM SPSS Statistics Grad Pack 28. For data analysis, descriptive and inferential statistics were used. Descriptive statistics were based on demographic data, and inferential statistics were used to see relationships and differences. The primary descriptive analysis was carried out to calculate frequencies and percentages. Inferential statistics: the Chi-square or Fisher exact tests were applied to test the contingency tables and the t-test for the differences in the arithmetic data between the two groups.
RESULTS
Thirty-five patients were randomized, 17 (48.6%) in the control group and 18 (51.4%) in the treated group. The age group was 50 ± 13 years (median = 49 years; minimum = 18 years; maximum = 71 years). The age distribution between the Control and Treated groups was not statistically different (Student t-test; p = 0.853). The distribution of age groups and Gender between the Treated and Control groups was homogeneous (for age, Fisher exact test; p = 0.740) (for gender, Fisher exact test; p = 1.00) (Table 1).
There were no pregnant cases, and there was no liver disease reported. Between the Treated and Control groups, there was an even distribution of cardiovascular disease (Fisher exact test; p = 0.738) and diabetes mellitus (Fisher exact test; p = 0.228). Homogeneity was confirmed between the groups in the case of neurological or neuromuscular disease (Fisher exact test; p = 1.00), the presence of immunodeficiency, including the human immunodeficiency virus (HIV) and lupus (Fisher exact test; p = 0.486), the distribution of renal disease (Fisher exact test; p = 0.486), and the distribution of chronic lung disease (Fisher exact test; p = 0.603) (Table 2).
In addition, there were no malignancy cancer cases in the Treated and Control groups. There was a significant association between the age group and the frequency of cardiovascular diseases (Fisher exact test; p = 0.018), being more frequent in the ≥ 50 years old group than in the younger group. No other associations were found.
In Table 3, a statistically significant association between the group and the Clinical Categorization was found (Fisher exact test; p < 0.001), more frequent in the higher categories in the Treated group than in the Control group. The clinical categorization significantly decreases over time (p < 0.001). However, this improvement was very close but not significantly different comparing the two groups (p = 0.058). The interaction between group and time was insignificant (p = 0.051). Despite the slight margin to significance, statistically, there was a failure to reject the null hypothesis (Table 4 and Table 5, and Figure 2).
There was a statistically significant association between the group and the shortness of breath (dyspnea) (Fisher exact test; p < 0.001), being more frequent in the Control group than in the Treated group. There was no statistically significant association between the group and fever (Fisher exact test; p = 0.757). There was a statistically significant association between the group and the respiratory rate > 30/min (Fisher exact test; p < 0.001), more frequent in the Treated group than in the Control group.
There was no statistically significant association between the groups and the blood oxygen saturation ≤ 93% (Fisher exact test; p = 0.864). There was a statistically significant association between the groups and the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) <300 mmHg (Fisher exact test; p = 0.007), being more frequent in the Treated group than in the Control group. There was a statistically significant association between the groups. The increase of >50% of pulmonary infiltrates between 24 and 48 hours of evolution (Fisher exact test; p = 0.001) was more frequent in the Control group than in the Treated group. There were no adverse events related to the plasma transfusion reported in this trial.
There was a statistically significant association for the diagnosis of respiratory failure (Fisher exact test; p < 0.001), found more frequently in the Treated group than in the Control group. There was no statistically significant association between the groups and the Diagnosis of Sepsis (Fisher exact test; p = 0.723). There was no statistically significant association between the groups and the diagnosis of septic shock (Fisher exact test; p = 0.336). There was a statistically significant association between the groups and the other adverse event observed (Fisher exact test; p = 0.001), more frequent in the Treated group than in the Control group (Table 6).
There was a statistically significant association between the groups for the qualitative PCR results (Fisher exact test; p = 0.010), being more “Positive” in the Control group than in the Treated group. The number of white blood cells (WBC), polymorphonuclear leukocytes (PMN), and D-Dimer means were significantly higher in the Treated group when compared with the Control group until day-six follow-up inclusive. The D-Dimer mean was significantly higher in the treated group when compared with the Control group during the six-day follow-up. The prothrombin time (PT) was significantly higher in the Control group than in the Treated group. The PT and partial thromboplastin time (PTT) means were significantly higher in the Control group when compared with the Treated group until day six (included) (Table 7).
Descriptive statistics Dependent variable: Clinic categorization
Discussion
This is the first randomized clinical research for convalescent plasma in the Dominican Republic. Demographics of the sample were similar and both the Control and the Treated groups recovered from COVID-19. The most significant advantage of CP therapy was obtained in the first six days, where the improvement in clinical categorization was significantly faster. However, at the end of the study follow-up, on day 28, both groups had satisfactory recovery. The therapy in both groups was significant, but the group that received CP did not reach statistical significance, with a p-value = 0.051. This result is so close that it would be understandable to argue that the sample size did not allow rejecting the null hypothesis. Still, it is evident that there is a perceptible benefit of the therapy.
The CP therapy-provided Control presented significant improvement in COVID-19 signs and symptoms. COVID symptoms include an increased appearance of dyspnea, defined as the sensation of shortness of breath, of >50% of pulmonary infiltrates between 24 and 48 hours of evolution, positive qualitative PCR results, and the elevated values of the prothrombin time (PT) and partial thromboplastin time (PTT) test were all significant findings in the Control group.
To suggest a tendency as opposed to chance for the advantages of CP as a coadjutant in the therapy of COVIV-19, a higher population of the study is also required. The purpose of inferential testing was not to reject this trial's null hypothesis.
These results demonstrate that the CP therapy from patients recovered with COVID-19 does not represent an additional risk for patients. It also makes a case for more research in an early infection for hospitalized—but not severe—cases. This prospective study serves as a pilot to propose an investigation with a representative sample where the benefits of this therapy's effects can be evaluated. To close the technological gap between developed and developing countries, while it is necessary to respond to economic needs, there is also a need for a vanguard culture in health education. In this sense, this research was impacted by a poor response from the call of volunteers and patients who meet the study's inclusion criteria.
The study's inclusion criteria followed the expected population that would benefit the most from the therapy to be evaluated. For this reason, it was challenging to find admitted patients without severity criteria. Even though audiovisual and digital promotion were carried out to obtain donors and volunteer patients, the enrollment of patients in this study was disappointing.
The decision was made to close the study with a sample smaller than expected, with a smaller number of individuals willing to participate than expected. Study results show that the Dominican Republic requires health promotion for tissue donation policies, specifically in the context of blood components and unsatisfactory blood availability and donations.
Conclusion
Difficulties in assembling patients compliant with inclusion criteria dwindled sample size. There was no statistical difference between the standard management protocol for early non-severe patients infected with COVID-19 and adding CP therapy to the treatment in the Dominican Republic. Without relevant adverse effects and events, both groups recovered, identifying CP therapy efficacy. This pilot trial proves that blood donation in the Dominican Republic requires government promotion, because its short supply may become a public health crisis in disaster preparedness or future pandemics.
REFERENCES
1.
van Griensven J, Edwards T, Gallian P; Ebola-Tx Consortium. Convalescent plasma for Ebola virus disease. N Engl J Med 2016;374(25):2500. [CrossRef]
[Pubmed]

2.
Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: Lessons from past coronavirus outbreaks. Clin Microbiol Infect 2020;26(10):1436–46. [CrossRef]
[Pubmed]

3.
Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24(1):44–6. [CrossRef]
[Pubmed]

4.
Farhat RM, Mousa MA, Daas EJ, Glassberg MK. Treatment of COVID-19: Perspective on convalescent plasma transfusion. Front Med (Lausanne) 2020;7:435. [CrossRef]
[Pubmed]

5.
6.
Sullivan HC, Roback JD. Convalescent plasma: Therapeutic hope or hopeless strategy in the SARS-CoV- 2 pandemic. Transfus Med Rev 2020;34(3):145–50. [CrossRef]
[Pubmed]

7.
Thijssen M, Devos T, Ejtahed HS, Amini-Bavil-Olyaee S, Pourfathollah AA, Pourkarim MR. Convalescent plasma against COVID-19: A broad-spectrum therapeutic approach for emerging infectious diseases. Microorganisms 2020;8(11):1733. [CrossRef]
[Pubmed]

8.
Barone P, DeSimone RA. Convalescent plasma to treat coronavirus disease 2019 (COVID-19): Considerations for clinical trial design. Transfusion 2020;60(6):1123–7. [CrossRef]
[Pubmed]

9.
Roback JD, Guarner J. Convalescent plasma to treat COVID-19: Possibilities and challenges. JAMA 2020;323(16):1561–2. [CrossRef]
[Pubmed]

10.
Psaltopoulou T, Sergentanis TN, Pappa V, et al. The emerging role of convalescent plasma in the treatment of COVID-19. Hemasphere 2020;4(3):e409. [CrossRef]
[Pubmed]

11.
Protocolo para el diagnóstico y tratamiento del coronavirus (COVID-19). Santo Domingo: Ministerio de Salud Pública; 2022. [Available at: https://repositorio.msp.gob.do/handle/123456789/1725]

SUPPORTING INFORMATION
Acknowledgments
This research was carried out thanks to funding from INIEICONT and UASD, supported by the Dominican Republic MSP and the HGPS. All authors, donors, and patients were volunteers in this research. Professor Guarionex Gomez (Statistics, UASD) and Dr. Ignacio Alvarez (Statistics-Madrid) contributed to the statistical analysis. Oskuary Jimenez and Luis Bido were from INIEICONT. Dr. Miguel Nuñez (Infectious Diseases, Dom. Rep.) was a scientific advisor.
Author ContributionsAngiolina Camilo Reynoso - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Socrates Sosa - Conception of the work, Design of the work, Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dolores Mejia - Conception of the work, Design of the work, Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ludwig Frontier - Conception of the work, Design of the work, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Angel Diaz - Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rafael Montero - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Angiolina Camilo Reynoso et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.